
What is the name of the group with 8 valence electrons?Ītoms of neon (Ne), argon (Ar), krypton (Kr), and xenon (Xe) have 8 valence electrons. Examples include neon (Ne), argon (Ar), and krypton (Kr). Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). Examples include hydrogen (H), lithium (Li), and sodium (Na). What row has 8 valence electrons?Ī: Any element in group 1 has just one valence electron. Atoms tend to form bonds so that they have 8 valence electrons and become more stable. These elements are nonreactive, or stable. What elements has 8 valence electrons?Ītoms of neon (Ne), argon (Ar), krypton (Kr), and xenon (Xe) have 8 valence electrons. The two elements that most commonly fail to complete an octet are boron and aluminum they both readily form compounds in which they have six valence electrons, rather than the usual eight predicted by the octet rule. What elements dont have 8 valence electrons? Do all elements have 8 valence electrons?Ī: Any element in group 1 has just one valence electron. The only exception is helium, which has just two electrons.
#Krypton valence electrons full#
Their outer energy levels are full because they each have eight valence electrons. Noble gases are the least reactive of all known elements. Which of the following noble gases does not have eight valence electrons? Which of the following has 8 valence electrons?Ītoms of neon (Ne), argon (Ar), krypton (Kr), and xenon (Xe) have 8 valence electrons. Which of the following does not have 8 valence of electrons?Ĭa+ does not have 8 valence electrons. What is the name of group 8 on the periodic table?.What group name has 8 valence electrons?.Which rows can have more than 8 valence electrons?.What is a set of 8 valence electrons called?.What group has 8 valence electrons and satisfies?.
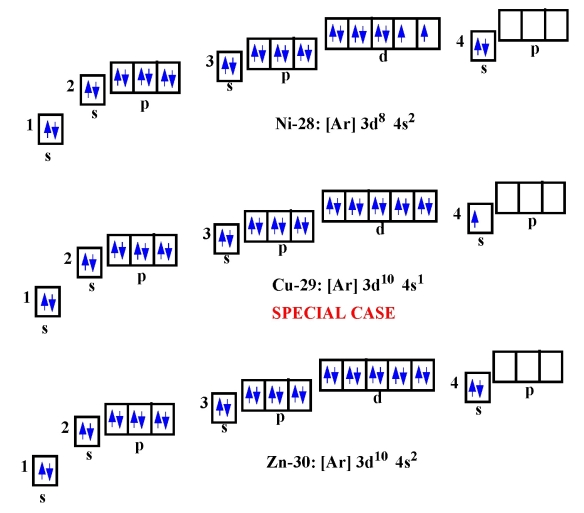
What are the 3 exceptions to the octet rule?.What Ion does not have 8 valence electrons?.Can an element have 9 valence electrons?.Which noble gas does not have 8 electrons in the highest energy level?.Which noble gas does not have an octet?.
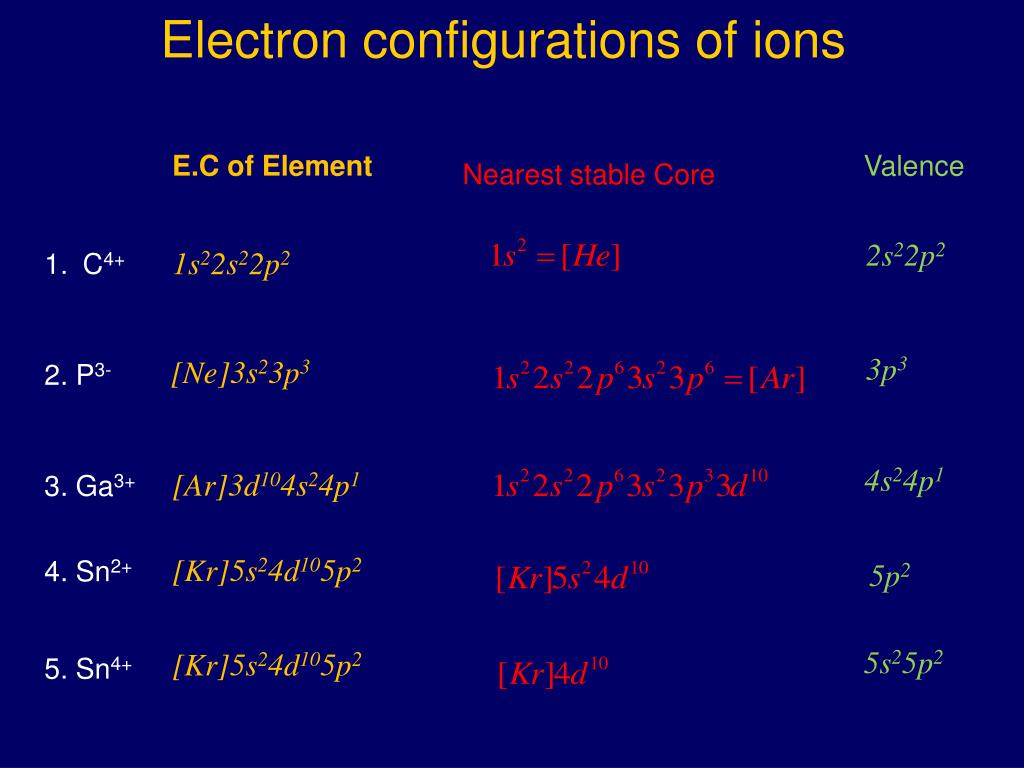


 0 kommentar(er)
0 kommentar(er)
